 |
| Image 1: To eat or to diet, what's worse? |
Even "healthy" weight cycling turns out to be profoundly unhealthy!
Now, the unfortunate news first: We are, as so often dealing with a rodent study - one that was done conducted with 80(!) 3-months old C57BL/6 mice. "Wow! 80 mice? That's plenty!" Yeah, initially it may sound like that, but in view of the fact that their number was decimated every 8 weeks, there would not have been the necessary 4x8 rodents left at the end of the 24-week study period for the final evaluation of the four experimental groups, which were
- standard chow (SC; 15kJ/g) - rodents in this group received the standard chow (76% energy from carbohydrates, 14% energy from protein, and 10% energy from fats) for the whole study period
- high fat diet (HF; 21kJ/g) - rodents in this group received a fattening hypercaloric diet (26% energy from carbohydrates, 14% energy from protein, 50% energy from animal lard and 10% energy from soy bean oil
- SC ↔ HF - rodents in this group received standard chow for the first 8-week cycle, high fat diet for the 2nd 8-week cycle and standard chow for the third and last 8-week cycle
- HF ↔ SC - rodents in this group received high fat diet for the first 8-week cycle, standard chow for the 2nd 8-week cycle and high fat for the third and last 8-week cycle
- the high school football player who turns to a sedentary lifestyle and bad eating habits when he goes to college, is partying all night, bear pizza, etc. eventually, he realizes he got fat, and diets again (SC ↔ HF ↔ SC) and
- the obese kid who eventually turns to physical culture, works out, eats health and loses weight, when he starts college, to then fall back into his old bad habits and starts letting himself go, when he marries and has kids (HF ↔ SC ↔ HF)
[...] after three consecutive WC [weight cycles], the reduction of BM is less marked during the SC cycle, as well as the increase of BM is more prominent during HF cycle (Barbosa-da-Silva. 2012).Now, we probably would not have had to do a 24-week rodent study to know that, right? Right! Notwithstanding, though, the beauty of working with rodents - instead of Biggest Losers, for example - is that they usually don't complain much when you slaughter them, so that the scientist could not only measure the serum leptin (figure 2, left), triglycerides, cholesterol, insulin and glucose levels, but also count the number and measure the size of the adipocytes in their visceral fat pads.
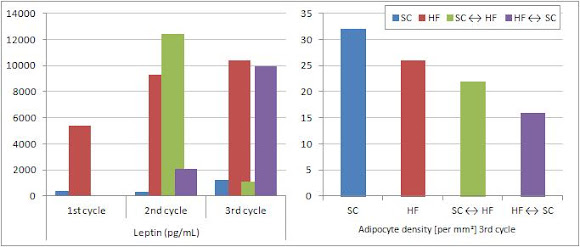 |
| Figure 2: Leptin expression and adipocyte density per area of adipose tissue mass after the 1st, 2nd and 3rd weight gain/loss cycle (data adapted from Barbosa-da-Silva. 2012) |
Adipocyte morphology, leptin expression, fat pad restructuring and body fat that sticks
Apropos significance, you ay remember from the "previously mentioned post" on this issue that one of the currently discussed hypothesis that could (at least partly) explain why formerly obese people are not just having a really hard time to lose weight, but also, and often even more so, to keep that weight off, relates to what I have previously labeled "relative leptin defiency" (too little leptin production per adipose tissue mass) or, and this would be an alternative hypothesis, "leptin resistance" (more than enough leptin in the blood, but the signal transmission does not work).
The first thing we can say based on the data Barbosa-da-Silva acquired on the absolute fluctuations of leptin in the blood of the rodents (figure 2, left) ist that previously made conclusions about the effects of weight gain, weight loss and energy intake on leptin, like
- weight loss and fasting are associated with reduced leptin levels,
- weight gain is associated with an increase in leptin concentration
- chronically increased leptin can lead to leptin resistance
- meals and according to meal composition or short-term swings in energy balance such as fasting or overfeeding induce swings in systemic leptin levels
 |
| Figure 3: Leptin levels in serum per body fat (left), leptin expression in adipose tissue (middle), and sectional area of adipocytes of the different groups (based on Barbosa-da-Silva. 2012) |
Some food for thought - Though not directly related to the topic, there is one thing pertaining to the heavily debated "CLA post" from last week (cf. "CLA Destroys Body Fat"), I want to mention. If we assume that the CLA-induced adipose tissue apoptosis Kim et al. observed in their recent study is as rodent-specific as the natural death and rejuvenation of adipose tissue Cinti et al. observed in the study I cite relating to the limited adipose tissue growth in rodents, this would not just indicate that taking copious amounts of CLA would not help to reverse the damage you may have done during previous "diets", but could also explain why conjugated linoleic acid supplements don't work in humans (or horses; see yesterday's news).
In combination with the leptin overshoot (+153%) in the "former football players on their college binge", this data would suggest that we are not dealing with "relative deficiency" and "leptin resistance" but rather with a complex mixture of both, where the latter is probably a result of repeated overshoots like the one we see in the SC ↔ HF group after their first high fat feeding cycle (2nd cycle, 154% elevated leptin levels).Now this segues directly into the allegedly somewhat counter-intuitive conclusion that anything that soothes the raging inflammation in your fat cells may ameliorate the downstream detrimental effects on glucose and lipid metabolism, but will, on the other hand, help your fat cells to survive or maybe even proliferate in amidst the TNF-alpha induced cytokine storm (Prins. 1997), which would otherwise kill them. Now with the current paradigm of "inflammation = bad" this may sound hilarious. In the the end, it does yet only echo the title of a 1999 paper by Hube and Hauner, "The role of TNF-alpha in human adipose tissue: Prevention of weight gain at the expense of insulin resistance?" (Hube. 1999) and would provide us with a mechanistic explanation of several otherwise non-explicable phenomena such as the profound fat loss in rodents who lack the master antioxidant glutathion (see "Inflammation Is the True Fat Burner"),,, but as indicated: This is just some food for thought ;-)
Relative leptin deficiency, systemic resistance and now local differences?
And as if things were nor already complicated enough, there are also potentially important differences between circulating leptin levels and local leptin expression in isolated fat pads figure 3 (middle; compare data to figure 2, left, 3rd cycle). Thus, the drop in leptin levels upon "fasting" in the (SC ↔ HF, 2nd cycle and HF ↔ SC, 3nd cycle) is systemic, but does not reflect the expression of leptin in the intra-abdominal tissue. This stands in line with my previous dissertation on "relative leptin deficiency" and the differences between...
- intra-abdominal (easy to shed on a diet), and
- subcutaneous (esp. in the lower body compartment difficult to shed on a diet)
The same group is however living (now dead ;-) proof that the notion that you could diet today, look better tomorrow and then return to what has gotten you into misery before is not just illusive, but outright life-threatening. Since caloric restrictions, which are still at the heart of 99% of the mainstream diets, will probably magnify the amplitude (i.e. the up and down) of the yoyo effect and its negative metabolic consequences, it appears reasonable to assume that the yoyo-dieter will eventually be worse off than the "happy fatso" who has been eating whatever he wanted for all his life and dropped dead morbidly obese with a heart attack at 45. After all, it seems likely that he (or she!) will not even live to the 45th year before he falls victim to the very same fate and that after not despite, but rather because of all the temporary austerities... now, this may be like choosing between pest and cholera, and the third option, i.e. following the path of physical culture would alway be my first choice, but honestly, if I had to choose, I'd rather be the fatso who enjoyed his 45 years of pizza, pasta and chocolate pie than the frustrated yoyo dieter.
- Barbosa-da-Silva S, Fraulob-Aquino JC, Lopes JR, Mandarim-de-Lacerda CA, Aguila MB. Weight Cycling Enhances Adipose Tissue Inflammatory Responses in Male Mice. PLoS ONE 2012; 7(7): e39837.
- Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005; 46: 2347–2355.
- Hube F, Hauner H. The role of TNF-alpha in human adipose tissue: prevention of weight gain at the expense of insulin resistance? Horm Metab Res. 1999 Dec;31(12):626-31.
- Kim JH, Kim J, Park Y. trans-10,cis-12 Conjugated Linoleic Acid Enhances Endurance Capacity by Increasing Fatty Acid Oxidation and Reducing Glycogen Utilization in Mice. Lipids. 2012 Jul 11.
- Niesler CU, Siddle K, Prins JB. Human preadipocytes display a depot-specific susceptibility to apoptosis. Diabetes. 1998 Aug;47(8):1365-8.
- Prins JB, Niesler CU, Winterford CM, Bright NA, Siddle K, O'Rahilly S, Walker NI, Cameron DP. Tumor necrosis factor-alpha induces apoptosis of human adipose cells. Diabetes. 1997 Dec;46(12):1939-44.
- Zhu. Ncb5or in Fatty Acid Desaturation and Metabolic Diseases. Zhu Diabetes Research Group. University of Kansas School of Health Professionals. < http://www.alliedhealth.kumc.edu/school/research/zhu/more_info.html > retrieved July 22, 2012












0 comments:
Post a Comment