 |
| Image 1: One reason why IF works is that it breaks the unnatural constant and convenient availability of high energy food and the subsequent suppression of AMPK phosphorylation (img courtesy of foxsearchlight) |
The delicate balance between mTOR and AMPK, this was another result of our considerations, is oftentimes broken in these days of nutritional abundance, where the rebuild and repair mechanisms of AMPK hardly get a chance to control the growth processes a constantly elevated mTOR pathway is triggering. The forced feeding-breaks on an intermittent fasting regime break this rampantly anabolic cycle. They let AMPK come into it's own and allow for...
- ...broken DNA strands to be fixed, before their (re-)use results in cancerous growth (Habib. 2010),
- ...cancer and defect cells to initiate apoptosis, i.e. to kill themselves (Chen. 2011)
- ...life extension via reduction of dietary glucose to work (Schulz. 2007)
- ...fat to be used as a substrate (Hardy. 2002),
- ...inhibiting adipogenesis = fat cell differntiation (Lee. 2011)
- ...mitochondrial biogenesis to be initiated (Zong. 2002),
- ...muscular GLUT4 activity and thus glucose uptake to be restored/increased (Holmes. 1999),
- ...gluconeogensis in the liver to be suppressed (Rutter. 2003),
- ...much much more healthy stuff ;-)
AMPK may be non-anabolic, but that is not necessarily a bad thing!
 |
| Image 2: Who would you prefer to be? Canto (left), on a life-extension (low calorie) diet with chronically elevated AMPK levels, or Owen (right), who enjoys his bananas to the fullest without even knowing about their profound effects on mTOR expression? (image taken from an article in the Irish Medical Times) |
Moreover, studies on the effects of the AMPK inducing drug AICAR (cf. previous news on AICAR) suggest that the increase in mitochondrial oxidation may also lead to dangerously high levels of radical oxygen specimen (ROS) formation (Kim. 2006), of which you have learned only recently, that there is a fine line between the benefits of some vs. the deleterious effects of too much free radicals. Which brings up - yet again! - the issue of balance!
Reversing perspectives: "Low energy" as the norm
In the previous episode, we have also identified energy availability or, to be precise, the ratio of the high energy ATP (adenosine triphosphate) to the lower energy ADP (adenosine diphosphate; -7.3kcal/mol) and AMP (adenosine monophosphate; -10.9kcal), as a crucial determinant of AMPK activation. In one of the most recent reviews on the topic (Carlin. 2011), David Carlin and colleagues from the Imperial College in London state that
[...] the finding that ADP, as well as AMP, protects AMPK against dephosphorylation influences the way we look at the physiological regulation of AMPK. To the best of our current understanding, the concentration of ADP in mammalian cells is much higher (10- to 100-fold)In other words, under normal conditions ADP, i.e. the higher energy variety of the dephosphorylated ATP molecule, and not AMP is the main determinant of AMPK activity. And, and this is a pretty novel finding, both ADP and AMP do not actually activate 5' adenosine monophosphate-activated protein kinase (AMPK), but rather prevent it from being deactivated. While it may seem that this does not really matter, looking at things this way let's the "deactivation of AMPK by energy abundance" - a state we have accepted as a norm - suddenly look like the exception; and when we come to think of it, all the "diabesity"-related ailments an ever-increasing percentage of our society is experiencing can be tracked back to the reversal of norm (=AMPK phosphorylated = restore and repair using stored energy) and exception (=mTOR phosphorylated = build, grow, store) that is triggered by the persistent abundance of energy.
than that of free AMP, and so it is likely to be the main regulator of AMPK activity under normal energy-stress conditions. The extent of the tighter binding of ADP to AMPK, relative to MgATP, essentially offsets the higher physiological concentration of MgATP. An interesting possibility is that under most conditions AMPK is regulated by the ATP:ADP ratio through changes in Thr172 phosphorylation state. Under severe stress conditions, however, when the concentration of AMP might increase markedly, the additional allosteric activation mediated by AMP could act as a type of fail-safe device, ensuring that all AMPK substrates are maximally phosphorylated.
 |
| Image 3: This is the way "fast food" is supposed to look like |
What I am trying to say, here is that in human history, exercise or at least "movement" usually preceded nutrient availability. To facilitate that nature has equipped us with a compulsion to move most that is most prominent in anorexic patients, whose desire to "get going" is in part (another factor, these days, is obviously the hilarious calories-in-calories-out conception) mediated by the same mechanism that triggers the "food seeking behavior" in rats (Guisinger. 2003). With the neolithic age "food seeking" has become obsolete and with the advent of modern fast and convenient foods the movements we are making to avail us of the next (mostly sugary) snack or meal, whenever our body senses that the energy level is about to drop to normal (notice the change in perspective), takes us from the couch to the fridge and back... but I am digressing from the topic at hand, so let's get back to how intermittent fasting plays into that.
Intermittent fasting = resisting the urge to go to the fridge
Obviously your usual "walk" to the refrigerator is a definitive "no-no", when you are on in intermittent fast - or, to get back to the paleo metaphor, you are like Paleo Eve waiting for Paleo Adam to bring the "fast food" he is just chasing (the rabbit from image 3) home for you to roast it (I assume you will have read that doing the same with potatoes is not a good idea). While you are sitting there (on the couch or at the fire place, whatever you like better ;-) the majority of your 5' adenosine monophosphate-activated protein kinase will obviously stay in the same phosphorylated state it was, when you woke up this morning. This, in turn, brings up the question when / how the enzyme (AMPK) gets phosphorylated in the first place. A question you will probably be able to answer, if you read yesterday's news item on the effects of postprandial carbohydrate, leucine or carb + leu feeding on muscle protein synthesis.
 |
| Figure 1: Postprandial changes in AMPK activity (relative to fasted state) 0min, 90min and 180min post ingestion of a 4g meal and following supplementation with water (control), carbohydrate (CHO), leucine (Leu), or leucine + carbohydrate 135min after the ingestion of the meal (data adapted from Wilson. 2011) |
In spite of the relatively rapid "restoration" of AMPK phosphorylation in response to "not eating", sitting on the sofa, watching TV and worrying about when you can finally break the fast is not what you would expect to give your physique the edge you are probably trying to achieve.
 |
| Figure 2: Weight of fat pads in g/100g body weight in AICAR (intraperitoneal injections @ 0.7g/kg body weight) treated vs. control male Wistar rats after 4 and 8 weeks of treatment (data adapted from Gaidhu. 2011) |
| Figure 3: Inguinal mytochondrial density in AICAR (data adapted from Gaidhu. 2011) | Figure 4: Energy expenditure in kcal/h (data adapted from Gaidhu. 2011) |
Optimizing AMPK during the fast by exercise
From previous installments of this series you know that the current "fitness-oriented interpretation of intermittent fasting", as I would like to call it, prescribes exercise as an obligatory 2nd element of a body recompositioning scheme à la leangains.com. It probably does not take a rocket scientists to gather that the energy depriving character of exercise will deplete cellular ATP levels, increase the APD:ATP ratio and thus prevent the dephosphorylation of AMPK. But if this is in fact the case (and it is, cf. "Exercise is perhaps the most powerful physiological activator of AMPK", Richter. 2009 ;-), the next question would be: How on earth can you still build muscle, if exercise activates AMPK and AMPK reduces protein synthesis? Well, the answer is simple: It's the seesaw principle!
fast + exercise > AMPK up || rest + feed > AMPK down
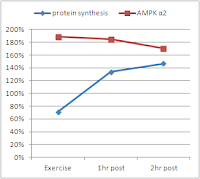 |
| Figure 5: AMPK and protein synthetic response (relative to basal levels) to resistance exercise in 7 men and 4 women (data calculated based on Dreyer. 2006) |
Exercise is in itself a trigger for muscle protein synthesis (Drummond. 2009)!
And if you remember the two posts on "glycogen-free muscle growth" related to the 2011 study by Camera et al. you will be aware that the phosphorylation of p70s6k and the subsequent increase in protein synthesis following resistance exercises does occur, even if you train in a glycogen-depleted state.
 |
| Image 4: Duong at the beginning and end of his 12-week intermittent fasting body transformation program; click here to read more |
With that, I will leave you hungry for more until tomorrow, where due to a national holiday, here in Germany, I will have time to continue my elaborations on AMPK's role in intermittent fasting and beyond. So, I would suggest, you come back tomorrow if you want more information on how to modulate AMPK and its effects on your physique by exercise, sleep and supplementation to make your intermittent fast (or whatever other diet you are following) even more productive!
update: Click here for part two...












0 comments:
Post a Comment